|
By: Josh Sloat
InnovatorMD MasterClass
In our upcoming MasterClass with InnovatorMD, we'll be discussing government grants and the strings that can come attached to your IP!
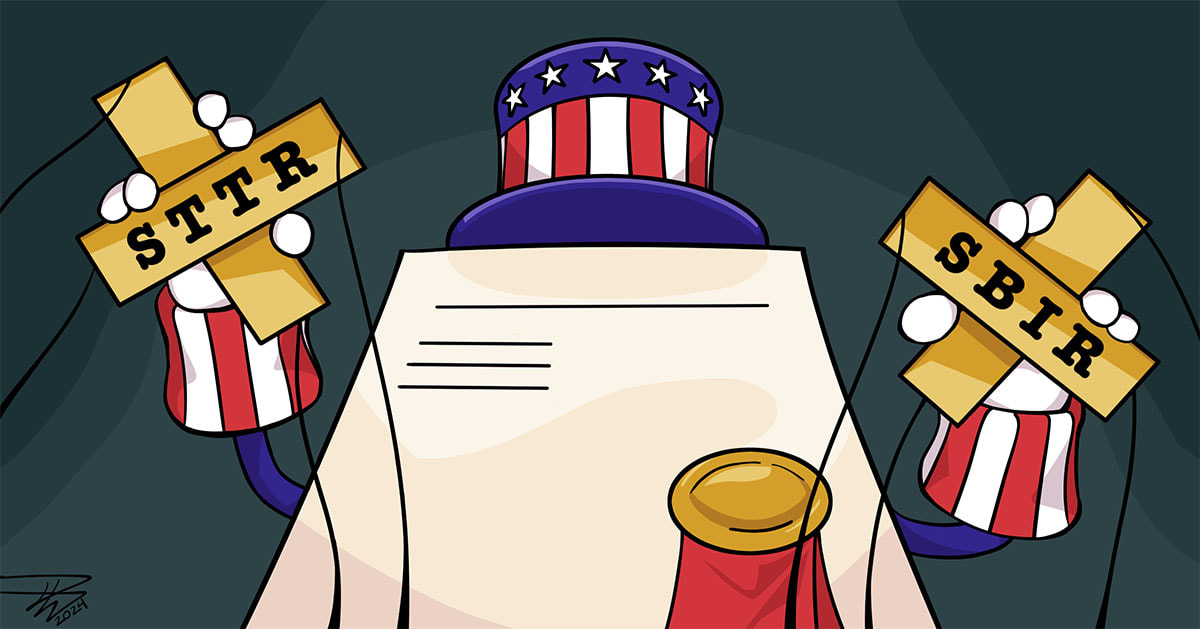
SBIR and STTR Grant Strings
The Small Business Technology Transfer (or STTR) and Small Business Innovation Research (or SBIR) grants are the largest source of early-stage capital for life science startups in the United States, combining to provide over $4 billion annually in support from federal agencies like the NIH. But like money from investors, friends, and families, these grants do still come with some serious strings attached and potential ramifications you need to be aware of.
About the Talk
Most of these things are manageable, but when considering government grants, you need to be aware of these gotchas so you’re going in with clear eyes and can manage the hooks in a way that doesn’t jeopardize your patent rights. To help you understand and navigate these complexities, we'll discuss:
About InnovatorMD
InnovatorMD's mission is to globally advance the work of physician innovators and entrepreneurs delivering solutions that revolutionize patient care. They are a group of physician innovators from various backgrounds with a single vision – to spread physician innovation to the rest of the world and to have a positive impact on patient outcomes and physician wellness while moving the healthcare industry forward.
2 Comments
By: Josh Sloat
What They See, They Will Be
In this month's podcast episode, we’re looking at one man’s efforts to immortalize the pioneering genius of African American inventors from the past 400 years. Our special guest today is James Howard, founder and Executive Director of the Black Inventors Hall of Fame.
James Howard is a college professor, design historian, entrepreneur, industrial designer, inventor, filmmaker, and restauranteur. He brings over 25 years of experience as a design professor and has authored a course on Design Thinking and Design History that explores the impact of design on society. As an accomplished Industrial Design educator and entrepreneur, Howard has lectured on the experience of Black American inventors. Howard himself is an extraordinary inventor with 20 patents, several of which we discuss, cover innovations that save people's lives daily. James' life work is now culminating in his mission of bringing a broad and detailed awareness to the important work of African American inventors, artists, and innovators who have inspired and forged ahead against tremendous odds and adversity. In creating the Black Inventors Hall of Fame, James hopes to have a place where kids of all ages and persuasions can go and be inspired to become the next generation of great scientists, engineers, doctors, and inventors. Because like the great inventor Lonnie Johnson says, “What they see, they will be.” Episode Overview
James and I cover a wide range of topics, including:
Mossoff Minute: Masimo v. Apple and the Media
In this month's Mossoff Minute, Professor Adam Mossoff discusses how there’s a pirate living in your Apple Watch and why the media’s coverage of Apple’s predatory infringement of Masimo's patents is missing the mark. We’re also publishing excerpts as short-form videos on Instagram Reels, YouTube Shorts, and TikTok.
How to listen
Patently Strategic is available on all major podcasting directories, including Apple Podcasts, Spotify, and Google Podcasts. We're also available on 12 other directories including Stitcher, iHeart Radio, and TuneIn, so you should be able to find us wherever you listen to podcasts.
Resources.
To further explore the topics discussed, see the following past episodes and resources:
Transcripts We're also providing computer-generated transcripts for improved accessibility and additional reference opportunities.
By: Josh Sloat
The Patently Strategic Podcast is back in action for the start of Season Four and we're leading off today with a very important conversation on Claim Construction! This is part two of an important three-part series and picks up from our Season 3 episode on Claim Strategies – which goes from claim basics all the way to effectively leveraging claim strategies. If you haven’t listened to that one yet or if any of this gets a little thick, we highly recommend you go back and give it a listen. There is not a more important concept to grasp in all of patenting. Claims define the boundaries of your intellectual property. The highly nuanced process that goes into crafting them is all the difference between a defensible, assertable, valuable property right – and an expensive vanity piece for your wall.
Valuable Lessons in Claim Construction
In this month’s episode, Kristen Hansen, Patent Strategy Specialist and creative claim construction worker here at Aurora leads a discussion, along with our all-star patent panel, delving into the world of claim construction. Claim construction is a process in which courts attempt to interpret the meaning and scope of the claims of a patent. It’s sort of like reverse engineering – or maybe more accurately reconstructing – what an inventor and their practitioner meant back when they drafted the patent application.
While your patent might not be tested in a court for many years, understanding the sometimes surprising language specifics and context traps while drafting now can help set you up for success later when defending your patent or attempting to stop an infringer. The words you choose now and the support you provide when drafting are your opportunity to help derisk the process of courts and juries later interpreting what you meant. And oftentimes, claim construction can be the KEY FACTOR in resolving disputes even before litigation, with the facts that come out of claim construction deciding the monetary value and payouts in settlements. In breaking this all down, Kristen and the panel discuss:
Kristen is joined today by our always exceptional group of IP experts including:
Mossoff Minute: Drug Price Controls
In this month’s minute, Professor Adam Mossoff discusses recently proposed regulations that would misapply Bayh-Dole provisions to impose march-in rights on patent-protected innovations and create price controls via compulsory licensing. This amounts to government seizure of private property and will do tremendous harm – especially to the life sciences – if implemented. We’re also publishing excerpts as short-form videos on Instagram Reels, YouTube Shorts, and TikTok.
Season Four Preview
It’s going to be another big year for patents and we couldn’t be more excited about the lineup we’re bringing you, including:
How to listen
Patently Strategic is available on all major podcasting directories, including Apple Podcasts, Spotify, and Google Podcasts. We're also available on 12 other directories including Stitcher, iHeart Radio, and TuneIn, so you should be able to find us wherever you listen to podcasts.
ResourcesRelated Episodes
Transcripts We're also providing computer-generated transcripts for improved accessibility and additional reference opportunities. Slides For the visual learners out there, we also like to make our presenter slides available for your reference.
By: Josh Sloat
Join Us in Houghton!
We're heading north to join our friends at Michigan Tech for their 2024 Innovation Week! Dr. Ashley Sloat will be delivering an important talk on Careful Contracting.
Husky Innovate hosts Innovation Week each year, which includes innovation and entrepreneurship presentations and panel talks that are open to Michigan Tech students, faculty, alumni, and the larger community. Hear from entrepreneurs and innovators from a variety of backgrounds. Innovation Week aims to educate, inspire, and encourage those leading innovations. Careful Contracting Talk
Learn strategies for careful contracting as you work with development and investment partners, and hear how investors evaluate your startup’s risk exposure. We'll discuss everything you need to know, from engaging with an engineering firm to ultimately pitching to an investment group. Topics include IP ownership, assignment from engineering firm inventors back to you, how to avoid the traps of viral IP, and what investors want to see in your pitch deck.
When: Thursday, Jan. 25, 12-1:30 p.m Where: MUB Ballroom B
By: Josh Sloat
We’re very excited to announce the winners of our 2023 RISE Award. Originally called the Relief for Innovative Startup Endurance Award, this is an annual award that we launched during the pandemic to help innovators struggling to endure the financial hardships that ensued. And an award that has given us so much joy, we’ve continued to offer through the years beyond, but now as the Recognition of Innovative Startup Excellence Award. For the winner, we provide a free provisional patent application or $5,000 in service toward a non-provisional. And this year, as in several years past, we’ve also worked with impressive runners-up on awards customized based on need – oftentimes helping out with things like patentability and landscape searches. Selection Process
Using a weighted matrix, our team scored applications based on the following criteria:
We can't say it enough, but the recipients are a truly gifted collection of entrepreneurs who will surely do great things. We couldn’t be more thrilled about the potential to be a part of their journey!
2023 WinnersThe first place, full $5,000 award goes to Absolute Concept Designs. Dustin Webb and his team at Absolute are developing a Hybrid Unmanned Aerial Underwater Vehicle (HUAUV) drone platform with the capacity to transition between air and water mediums and perform a number of different operations. At this time, we're unable to disclose anything more specific about the capabilities, but trust us – IT'S REALLY COOL! The platform would be utilized for various amphibious missions instead of sending a person into contested environments, resulting in saved lives of our Service Men and Women and increased security for the US and our Allies across the globe. The ACD team has serious experience in this space, having previously worked with the Department of Defense on the design of armored vehicles, underbody blast mitigation technologies, air-to-air missiles, cruise and surface-to-air missiles, fighter jets, helicopters, and drones. Our thought bubble. ACD's HUAUV is just the kind of impactful innovation, backed by the kind of DNA it takes to see through to market success. The development and protection of any technology that helps to keep US Service Men and Women out of unnecessary danger is a mission we’re proud to help facilitate in any capacity!! Being chosen as the winner of this year's Rise Award will allow our business to accelerate the development of our HUAUV (Hybrid Unmanned Aerial Underwater Vehicle) Platform. With Aurora's generous support and vast experience in the patent industry, we know that our HUAUV Platform is going to be well safeguarded against potential competitors, thus giving us a strong position as we enter the drone market. We’ve selected Rebel Cultures as our first runner-up, to receive a $2,500 patent services award. Rebel Cultures is on a mission to help the world overcome critical plant shortages by inventing a new, high-efficiency, lower cost method of plant production. Della Fetzer and her team are working on a lab-free method to efficiently propagate disease-free young plants. Their modular device stimulates the replication of thousands of young plants per cubic foot of production space!! This invention will allow plant producers in the forestry and agriculture industries to overcome their current barriers to producing enough young trees and crops to sustain our lives on earth. Founding Rebel Cultures team members were leaders in commercial-scale laboratory plant production for years, where they learned the techniques, industry, and shortcomings of the current fastest way to make plants. Rebel Cultures is positioned to succeed because their team is leveraging knowledge and experience gained in the ornamental horticulture market to be the first prominent non-seed solution for the forestry industry amid a growing global seed shortage. Our thought bubble. We’re very excited about Della's mission and think it’s the sort of innovation that’s not getting nearly the focus it deserves, given how important it is to literally sustaining all life on the planet. Her team also clearly has the internal expertise to really make a difference. This is the biggest piece missing from the environmental movement and something we’re happy to get behind and support however we can. Developing a new way to produce plants isn't easy, but with support from our community, early adopters, and trusted allies like Aurora Consulting, it's happening. This RISE award is an honor and will allow Rebel Cultures to participate in the centuries-old tradition of securing IP to accelerate our positive impact across the greater agricultural industry. We've selected Sand Baggage as our second runner-up, receiving a free patentability search and analysis. Sand Baggage founder Michael Wahlstrom designed a neoprene bag with a clever locking mechanism that uses beach sand for securing valuables while at the beach. Michael's inventor story is an inspiring one about the power of self-bootstrapping when leveraging your own expertise. His company is 100% bootstrapped with zero debt, and he possesses all the core competencies required to take his product to market without the need to outsource a single function. Michael is a professional with over 20 years of experience in the apparel and accessories industry. He runs a creative design, cut, and sew consumer product studio specializing in handbags, leather goods, pet accessories, private label fashion apparel, and merchandising brand strategy. His background spans the entire product lifecycle starting with retail management, merchandising, wholesale, buying, product development, technical design, sourcing, manufacturing, construction, and finishing. Our thought bubble. Michael's invention is a very practical solution to a real world problem – and a great example of the kinds of solutions that can be informed by experiences in our wonderful Northern Michigan backyard. We’re beach dwellers in our spare time and have very much felt the insecurity that comes with leaving valuables behind on the beach while swimming or doing other water sports that require leaving high dollar items behind and untethered. Beyond that, we love gritty entrepreneurs who roll up their sleeves and find ways to innovate – and really appreciate how Michael is self-bootstrapping and leveraging his own talent stack to bring this product to market. We couldn’t be more thrilled to be a part of his innovation journey! Winning the RISE Award and working with the team at Aurora has been the greatest gift for my startup. As a finalist in various business pitch competitions, they have helped structure an invention protection strategy that has allowed me to focus on other facets of my business. We've selected Chefshare as this year's final runner-up, also receiving a free patentability search and analysis. Erin Eatough, PhD and her team at Chefshare are on a mission to help modern families reclaim their most precious non-renewable resource of time and bring peace, connection, and joy back into weeknight dinners. Hooray! They do this through a service that will offer heat and serve meals specifically designed for busy modern families, from local area chefs. The approach uses customer-provided preference data, paired with chef-prepared weekly menus to cluster and match meal orders in order to reduce the price point of private chef services, making it more accessible. Erin is a mother, psychologist, and start-up tech executive. She founded Chefshare after becoming fed up with the time-starvation in her own life as a working mother. After auditing her time, nightly dinner was the most consistent, daily demand - both cognitively and physically - getting in the way of spending more quality time during the busy week with her kids. After hiring a private chef, she realized firsthand the huge return on the investment of outsourcing not just the food but the deciding, inventorying, planning, cooking, and cleaning for nightly dinners. She founded Chefshare to make outsourcing this part of modern life more affordable and accessible for other families too. Our thought bubble. As parents who'd prefer to be playing with our kids or doing something else rather than cooking dinner, Chefshare really resonates with us. More broadly, so many households do not like or have enough time to cook (or learn to cook!) and if you can create a good meal that is healthy and affordable, that’s a big win. We also appreciate Erin's local, stepwise approach to scaling the business model. We're excited to be a part of Erin's innovation journey and can't wait to be one of her first customers! I am incredibly grateful for Aurora and the RISE award! This award is enabling me to get the foundational legal searches done for patentability potential in using technology to improve the lives of busy working families by outsourcing all things related to weekly family meals. Ashley and Josh not only have instrumentally done legal work to support this business but have supported me with encouragement to build something amazing! Past Winners
To learn more about past recipients and hear stories about their innovation journeys, check out our Patently Strategic Podcast episode where we interviewed winners from the 2021 RISE class.
By: Josh Sloat
From patenting classic board games like Monopoly and Battleship back in the 1930s to challenges with protecting modern innovations in areas like game development and VR, our experts are breaking down everything you need to know about patenting games so you don’t end up just rolling the dice when investing in protections for your entertaining innovations. As a bonus, in this month’s episode, we’re bringing you two dealers: Dr. David Jackrel, President of Jackrel Consulting, will be covering the physical realm of board games and toys. Kristen Hansen, Patent Strategist and software guru here at Aurora, will be covering all things computer and video games in the second half. David and Kristen are joined by our always exceptional group of IP experts. Two exceptional gamers who would never be regarded as NPCs:
David, Kristen, and the panel discuss:
Mossoff Minute: Inventor's Hall of Fame
Professor Adam Mossoff recently attended the annual Inventor’s Hall of Fame induction ceremony and for this month's Mossoff Minute, discusses two sets of inductees and their groundbreaking inventions of the mRNA platform and CRISPR gene editing technology. We’re also publishing excerpts as short-form videos on Instagram Reels, YouTube Shorts, and TikTok.
RISE 2023 Award Winners
In this episode, we're also announcing the winners of the 2023 RISE Award! To learn more about this year's RISE award winners including Absolute Concept Designs (Dustin Webb), Rebel Cultures (Della Fetzer), Sand Baggage (Michael Wahlstrom), and Chefshare (Dr. Erin Eatough), please read the full announcement.
Availability
Patently Strategic is available on all major podcasting directories, including Apple Podcasts, Spotify, and Google Podcasts. We're also available on 12 other directories including Stitcher, iHeart Radio, and TuneIn, so you should be able to find us wherever you listen to podcasts.
ResourcesRelated Episodes
Transcripts We're also providing computer-generated transcripts for improved accessibility and additional reference opportunities. Slides For the visual learners out there, we also like to make our presenter slides available for your reference.
By: Josh Sloat
Illustration: Declan Wrede
Join us November 16th!
Dr. Ashley Sloat has been invited to deliver this year's Patsnap Frontier Conference Keynote. This incredible online conference connects innovators and industry leaders from across a diverse array of technology sectors to share key insights and dive deeply into current trends. With thousands of online attendees each year, this annual event has evolved into a thriving hub for conversations about the future of innovation. This year, expert speakers will be discussing topics ranging from optimizing the integration of artificial intelligence and overcoming technical obstacles, to strategically leveraging intellectual property to drive innovation and pave the way for the future. Our keynote will explore the ongoing and impending impacts of AI in the IP world and the possible futures it could create, looking at both dystopian and utopian outcomes for the patent system.
Will AI Terminate or Save the Patent System?
Our news feeds are overrun with stories of how AI is profoundly reshaping the world at a breakneck pace and achieving results that were only recently the mere dreams (or perhaps threats!) of science fiction. But how will this now inevitable rise of the machines reshape our IP legal industry and the very property rights systems that undergird science, technology, and the modern innovation economy? Beyond fears of a dystopian employment outlook for agents, attorneys, and searchers, could AI actually invalidate the entire patent system once it’s no longer able to deliver on its constitutionally expressed goals? Or could super intelligence actually be just the kind of cybernetic hero from the future that the breaking system needs to save so many patents (and an unsustainable bar) from an unfortunate judgment day?
Learn more and register
The conference takes place online on November 16, 2023. Our keynote will start around 9:20 am ET. Registration is free! Learn more and sign up below.
By: Josh Sloat
In this month's podcast episode, we’re talking about claims – the fundamental building blocks of a patent. There simply is not a more important concept to grasp in all of patenting. As a former chief justice of the Federal Circuit once famously said, “The name of the game is the claim.” And in terms of what game you’re playing, the claims are where you separate the patents playing checkers from the patents playing chess. This is where your patent practitioner earns their money and as you’ll learn today, also where the most costly mistakes can be born. As David Cohen, a Patently Strategic regular, has said in the past, "Ninety percent of the mental exercise in drafting patents is in the strategy of looking around corners, anticipating the future, and trying to capture as many would-be infringers as possible.” How your claims are crafted is literally the difference between a patent being an intellectual asset and a worthless stack of paper. In this month’s episode, Ty Davis, Patent Strategy Associate here at Aurora, leads a discussion, along with our all star patent panel, delving deeply into:
Ty is joined by our always exceptional group of IP experts, including:
Mossoff Minute: Advancing America's Interests Act
This month's Mossoff Minute, featuring Professor Adam Mossoff, looks at the poorly named Advancing America's Interests Act and its potential impact on the ITC being able to block import of products that infringe on American patents. We’re also publishing excerpts as short-form videos on Instagram Reels, YouTube Shorts, and TikTok.
Availability
Patently Strategic is available on all major podcasting directories, including Apple Podcasts, Spotify, and Google Podcasts. We're also available on 12 other directories including Stitcher, iHeart Radio, and TuneIn, so you should be able to find us wherever you listen to podcasts.
ResourcesRelated Episodes
Related Reading Transcripts We're also providing computer-generated transcripts for improved accessibility and additional reference opportunities. Slides For the visual learners out there, we also like to make our presenter slides available for your reference.
By: Josh Sloat
Illustration: Declan Wrede
20Fathoms Know Your Worth: Intellectual Property
Join us for lunch at 20Fathoms on October 11th when we'll explore how to leverage patents as one of your startup's earliest and most important assets. When you're first starting out, you don’t have customers, you don’t have revenue, and you probably don’t have physical assets. What you do have are your intellectual assets. These fruits and labors of the mind are not just defensive tools for protection, but chips you can leverage on that glorious path to investment, physical assets, customers, and revenue. Constitutionally enshrined property rights like patents and copyrights allow you to define ownership boundaries around your inventions and creative works, much like the title for your home defines the boundaries of your physical property. And just like your physical property, your intellectual property can be used as leverage, transferred, sold, and leased. And at the earliest stages, these IP assets can get you access to the venture capital you need to scale or to build the bridge from intellectual to real capital and from the garage to the marketplace.
You'll learn about how to protect these key business assets while avoiding common mistakes in the context of an overall IP strategy in this lunch-and-learn presented by intellectual property expert, Dr. Ashley Sloat. We'll be covering intellectual property like trade secrets, trademarks, copyrights, and patents, as well as how open-source IP should be treated. We'll also touch on responsible engagement with employees and vendors, especially around contracting, and typical mistakes made with IP. This event is hosted by 20Fathoms in partnership with Aurora Patents. This is an in-person event, and there is no charge to attend. Lunch will be provided. Date and Time: Wednesday, October 11 · 11:30am - 1pm EDT Location: 20Fathoms | 10850 East Traverse Highway #Ste, 4400 Traverse City, MI 49684
By: Josh Sloat
It's impossible to overstate the importance of protecting our most important natural resource. And that's why we couldn't be more excited to announce that we're joining forces with AquaAction to provide patenting assistance for their vital mission to restore freshwater health in North America! Aqua's programs, like its Hacking Challenge, present incredible opportunities to help accelerate technologies that will both improve and protect our precious water ecosystems, as well as boost the local innovation economies they touch. Aurora will be providing patent coaching to all participants, as well as service-based awards for the top three finalists in the annual, bi-national AquaHacking Great Lakes Challenge. We are thrilled about this partnership, eager to see where our collective energies take this, and confident it will mean great things for the region and the sustainability of its most important natural resource. AquaAction
AquaAction is a Canadian and US registered charity established by the Fondation de Gaspé Beaubien in 2015, created to disrupt the status quo with innovative ideas and engage youth in the fight against the water-related climate crisis. AquaAction has developed three programs focused on water innovation and technology: the AquaHacking Challenge, AquaEntrepreneur and the AquaAction Alumni Community. Since its establishment, 28 innovative start-ups have been created across Canada.
AquaHacking Challenge for the Great Lakes
The AquaHacking Challenge is a tech innovation program focused on developing solutions to pressing freshwater issues within the Great Lakes watershed region. The program is open to American and Canadian post-secondary students and young professionals looking for a hands-on way to apply their talent and fight the freshwater crisis. The solutions developed today will help save our freshwater systems for generations to come.
The 2023 Challenge started in September and will run for 9 months. Participants will receive mentoring and expert coaching, have access to on demand content in innovation, entrepreneurship, leadership and be eligible for micro-credits and digital badging. The 2023 AquaHacking Challenge is the first of three that will run sequentially from 2023 to 2026. Michigan's Blue Economy
We're personally and professionally very excited about the potential for initiatives like Aqua's Hacking Challenge, the Fresh Coast Maritime Challenge, and related water-focused technology efforts around things like electric boating. When you combine the access to our natural assets, a strong desire to protect them, the collective background expertise of so many who've grown up around freshwater, and the startup support given by groups like 20Fathoms and institutions like NMC, there's no reason why Northern Michigan shouldn't establish itself as a leading innovator in the blue economy.
The AquaHacking Great Lakes Challenge is co-hosted by Northwestern Michigan College (NMC) and AquaAction. We are very proud to have been invited by NMC to collaborate on this bi-national program for the Great Lakes. This immense watershed region is home to 27% of the population of Canada and the US, accounts for 28% of our combined economic activity, and holds 21% of the world’s freshwater. The business case for solutions is clear and we welcome a new generation of innovators to lead the way towards a clean and healthy freshwater future for us all and future generations – thanks to their innovative ideas. |
Ashley Sloat, Ph.D.Startups have a unique set of patent strategy needs - so let this blog be a resource to you as you embark on your patent strategy journey. Archives
July 2024
Categories |















 RSS Feed
RSS Feed